Popular heartburn drug may have given millions of people cancer, found contaminated with cancer chemical
10/03/2019 / By Ethan Huff

Another Big Pharma drug is being pulled from pharmacy shelves after it was discovered that millions of people could have developed cancer from taking it.
Zantac and other ranitidine products are reportedly no longer available at CVS, which joined a handful other other retailers in suspending Zantac sales pending an investigation into “impurities” that could be linked to life-threatening side effects.
After both Canada and France issued official recalls of Zantac, the United States and the European Union decided to launch inquiries into the drug’s safety, which in turn has prompted some retailers to voluntarily stop selling Zantac until a conclusion is reached.
As usual, health authorities here in the U.S. claim that there’s “no immediate risk” from taking Zantac. But patients are being advised to contact their physicians to request prescription alternatives, if they’re available.
For more related news about the dangers associated with pharmaceutical drug use, be sure to check out ChemicalViolence.com.
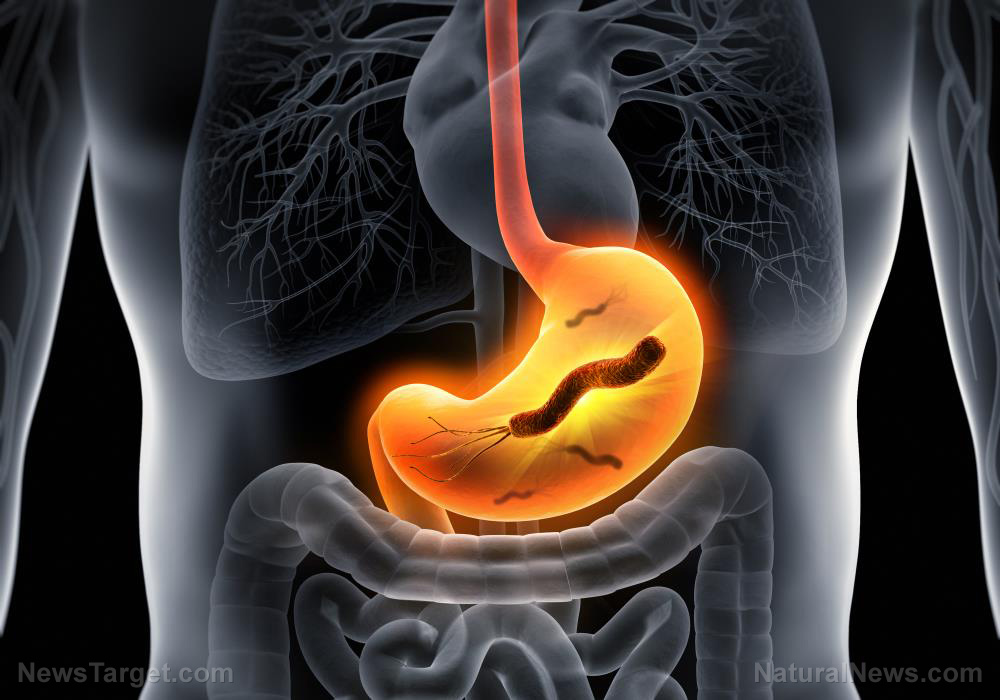
The FDA says cancer-causing chemicals in Zantac are “low,” so no mandatory recall is being issued
According to both the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), Zantac and other drugs that contain ranitidine are also contaminated with a chemical compound known as N-nitrosodimethylamine, or NDMA, which is classified as a probable human carcinogen.
What this means, of course, is that taking drugs with NDMA in them could cause users to develop cancer over time, which is why some countries have mandated a recall. But the FDA insists that levels of NDMA in Zantac are “low,” and has thus not issued a mandatory recall.
But consumers are still being advised to seek alternatives, or to simply stop taking Zantac and all other ranitidine drugs. Keep in mind that Zantac is also linked to causing depression as well, which is another reason not to take it.
“Zantac brand products and CVS brand ranitidine products have not been recalled, and the FDA is not recommending that patients stop taking ranitidine at this time,” CVS revealed in a statement following its decision to suspend the sale of both Zantac and CVS Health brand ranitidine.
The company added that while a mandatory recall was not issued, CVS has chosen to cease sales until further notice “out of an abundance of caution.”
This decision by CVS came after Walgreens, Walmart, and Rite Aid had already made this same decision themselves in an effort to protect consumers against potential health risks.
A little bit of heartburn is probably better than a cancer diagnosis, wouldn’t you say?
Meanwhile, Bangladesh, where some ranitidine products are manufactured, has issued a temporary ban on the import of these drugs. Bangladesh will also stop producing and selling ranitidine products until a conclusion is reached about their safety.
Sandoz, a drug company owned by the more popularly known Novartis, has similarly recalled “several batches of its ranitidine-containing medicines” from a number of European countries, including Austria, Belgium, Croatia, the Czech Republic, Denmark, Finland, Germany, Hungary, North Macedonia, Portugal, Slovakia, Slovenia, Sweden, and Switzerland.
GlaxoSmithKline (GSK), the company that originally developed Zantac, was approached by BBC News and asked to comment about the situation, to which it responded that it has stopped distributing its generic version of Zantac, as well as recalled all ranitidine products from India and Hong Kong.
“They have very little knowledge of cancer and if they cannot even get heartburn medication right how can they do anything else right,” wrote one Blacklisted News commenter about the situation.
“Cancer is a much more lucrative business than heartburn relief, and if the meds give rise to a few more cancer patients well, mission accomplished,” commented another.
Be sure to check out this article, which reveals how Zantac, Prilosec, and various other heartburn drugs are linked to an increased risk of death. And read PrescriptionWarning.com for more news about dangerous medications.
Sources for this article include:
Tagged Under: Big Pharma, cancer, chemicals, CVS, drug, FDA, heartburn, medications, pharmaceutical, pharmacies, Prescription drugs, ranitidine, Recall, toxic ingredients, toxins, Zantac
RECENT NEWS & ARTICLES
COPYRIGHT © 2017 FDA NEWS